New production capacity needed
Dr. Reckeweg & Co. GmbH has a production site in Bensheim for homeopathic medicinal products in solid, semisolid and liquid form.
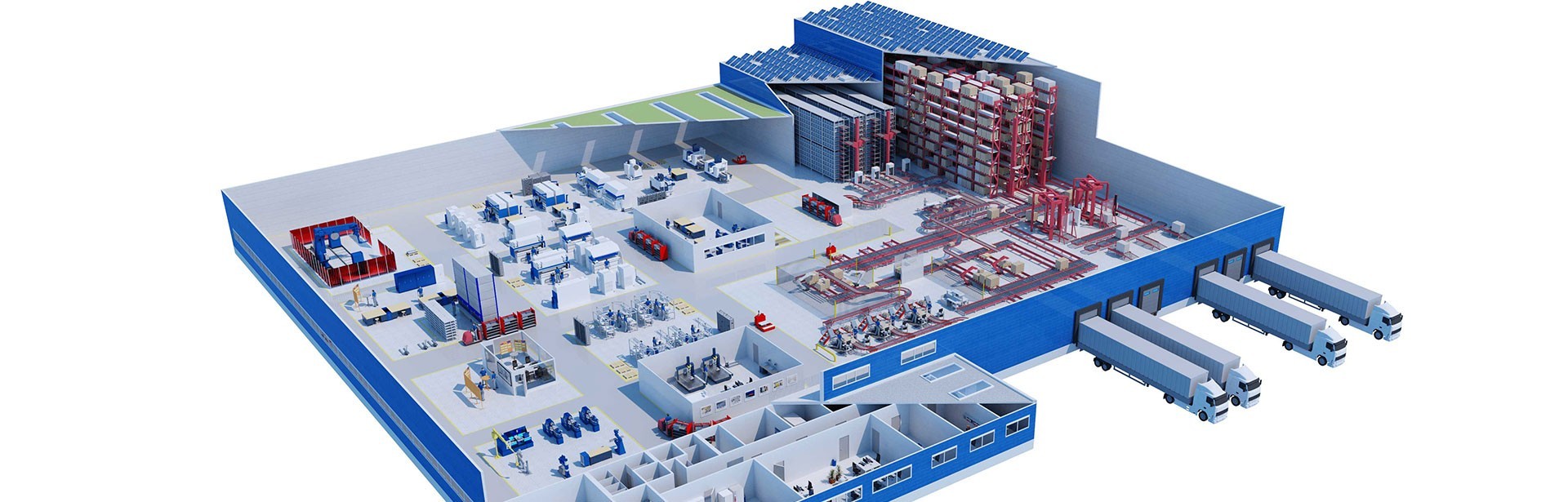
The steadily growing demand for homeopathic medicines in Germany and abroad resulted in Dr. Reckeweg needing to expand its production capacity. A new production building with an adjoining high-bay warehouse was constructed for this purpose at the Bensheim site, designed for short-term storage of large quantities of raw materials and products.
New production capacity in the clean room was needed to relocate parts of the existing production operation as well as new medicinal cannabis products.
The RCW3 project represents Dr. Reckeweg’s largest single investment to date. We commissioned io as lead consultant to make this highly complex investment project manageable.
Successful qualification of all involved parties
io supervised the new construction of production and logistics areas with clean rooms on a building area spanning around 11,000 m². The company drew on its many years of lead consulting experience in production and logistics to implement the project from concept design to go-live, including GMP qualification of all the involved parties.
io developed a controlled substance security concept for the production of cannabis products classified as narcotics (controlled substances). This involved implementing additional strong rooms, sophisticated intruder alarm systems, and camera surveillance in the clean rooms. Production logistics functions are integrated into the new high-bay warehouse with 4,000 pallet spaces, and previously outsourced warehouse capacities were brought back in-house.
Implementation and comprehensive GMP qualification and monitoring were successfully completed and ratified by the regional council responsible for the approval of new pharmaceutical production facilities. The production facilities were then migrated from the former plant. Production is already under way at the new site.
Key Facts
Client
Dr. Reckeweg has been developing and producing homeopathic combined pharmaceuticals since 1947. The company moved its operation to Bensheim in 1955 and employs 212 people there. It supplies products from its wide-ranging portfolio to 40 countries across the world.
The challenge
- Produce several dosage formats in a single production facility (liquid oral, solid oral)
- Establish production for the medicinal cannabis sector
- Steadily growing demand for homeopathic medicines at home and abroad
- Avoid contamination between material flows
Solution
- Strategic consulting on production processes and technologies
- Planning of a process chain for tablet and pill production
- Development of a controlled substance security concept for the production of cannabis products classified as narcotics (controlled substances)
Bird's eye view of the Dr. Reckeweg production facility