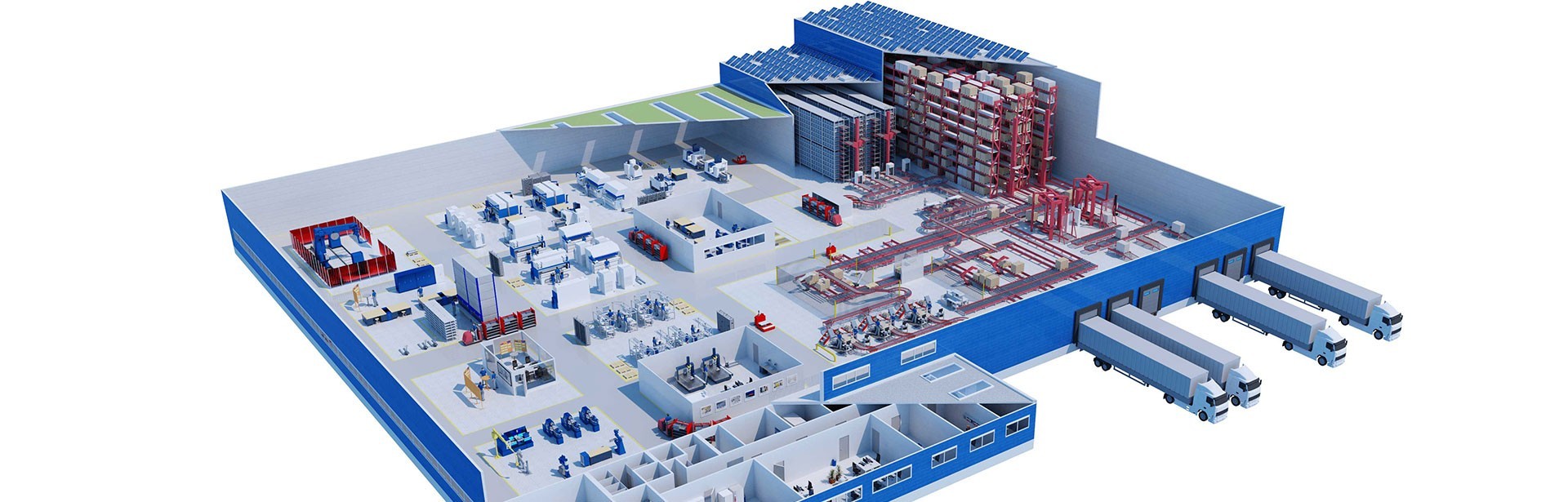
Support for production through optimal logistics processes
LTS commissioned the construction of a new logistics center at its Andernach facility to meet growing and constantly changing production requirements. The purpose of the new build was to support the sustained growth in production with optimal logistics processes. Concentrating the logistics processes at the facility would enable quick and flexible responses to production requirements.
The growth of the company and, in this context, the importance of the investment in the expansion of the production site for the development, manufacturing and logistics of surgical instruments are at the forefront.
Right from the start, optimizing all processes at the logistics center was a key priority to us as a pharmaceutical manufacturer. All processes follow the LEAN approach and support our work with streamlined interaction between production and logistics.
Key Facts
Client
Lohmann Therapie Systeme AG (LTS)
Founded in 1984, LTS is the global market leader in developing and manufacturing transdermal systems and oral active ingredient films, effective against Alzheimer’s, Parkinson’s and other diseases.
The challenge
- Increased demands on production
- Strict pharmaceutical authority requirements for the logistics center
- Linking logistics processes, buildings and regulatory requirements
Solution
- Optimizing internal logistics processes
- Controlling transport and handling steps through SAP-supported mobile data capture and bar code identification
- Integrating warehouse management and material flow control with SAP WM into LTS’s central SAP ERP system
- High-security protection for sensitive pharmaceuticals with controlled storage at 15‑25°C
- GMP and GSP
The building will be one of the first industrial buildings in Germany to meet the stringent requirements of the KfW Efficiency House Standard 40, consuming 60% less primary energy than comparable properties.
The core design of the building is to effectively blend in with the nature of the region
io’s production, logistics, quality assurance and IT experts collaborated closely from the concept phase on, to design a building that would enable an optimized flow of goods.
During the course of the project, all existing processes were scrutinized, reviewed and supplemented by new process flows, as and where necessary. To name just one example, all internal transports and handling steps are now controlled by SAP-supported mobile data capture and bar code identification. The entire warehouse management and material flow control of the automatic pallet high-bay warehouse was also integrated into LTS’s central SAP ERP system using SAP WM.
Pharmaceutical authorities across the world impose strict regulatory requirements on logistics centers. io took this key aspect into account in all the planning aimed at optimizing processes.
LTS invested a total of €16 million in the construction of its new logistics center at the Andernach facility.

The automatic pallet high-bay warehouse was also integrated into LTS’s central SAP ERP system using SAP WM.